Research | Tick Bytes
A Clinical Data Repository
The Problem
There is an urgent need for better Lyme disease treatments, with cases hitting a record annual high of 475,000 in 2019.
Studies show that the recommended Lyme disease treatment protocols fail patients somewhere between 10% to 36% percent of the time.
No Data Found
The National Institutes of Health allocated only 0.24% ($420,558) of its total Lyme research budget for human treatment studies from 2013 to 2020.
Primary care physicians are now trapped in a treatment-advice vacuum, with no effective strategies for helping the growing number of chronically ill tick-borne-disease patients
Our Plan to Fix it
Collect anonymized patient clinical data (symptoms, lab results, treatment effectiveness, etc.)
Organize the patient data in a secure, privacy-protected database that we’re calling Tick Bytes
Have researchers analyze the data to find ways to improve diagnoses and treatments
GIFS Via GIPHY
Our Plan to Fix it
Collect anonymized clinical patient data (symptoms, lab results, treatment effectiveness, etc.)
Organize the patient data in a secure, privacy-protected database that we’re calling Tick Bytes
Have researchers analyze the data to find ways to improve diagnoses and treatments
GIFS Via GIPHY
It Begins in the Clinics
Our community sites will collect data from the nation’s top clinics. These sites will be placed in states with a high incidence of tick-borne diseases.
We’re partnering with some of the nation’s top universities, beginning with Harvard, to collect clinical data.
After we fine-tune our data collection and storage processes, we’d love to add global sites into our network of clinics.
Why Clinical Sites...
- COMMUNITY PHYSICIANS
We need more funding to expand to other states. If you’d like to help make a Site in NJ a reality, you can donate to us here!
We need more funding to expand to other states. If you’d like to help make a Site in NY a reality, you can donate to us here!
We need more funding to expand to other states. If you’d like to help make a Site in CT a reality, you can donate to us here!
We need more funding to expand to other states. If you’d like to help make a Site in CA a reality, you can donate to us here!
We need more funding to expand to other states. If you’d like to help make a Site in MD a reality, you can donate to us here!
We need more funding to expand to other states. If you’d like to help make a Site in WA a reality, you can donate to us here!
- ACADEMIC MEDICAL CENTERS
This is our first academic Site! More information about it will be updated here when it’s available.
This is our second academic Site! More information about it will be updated here when it’s available.
We’re still reaching out to potential academic Sites. When we find the right fit, it’ll be posted here.
We’re still reaching out to potential academic Sites. When we find the right fit, it’ll be posted here.
How Clinical Data Gets Collected
questionnaire
Jane and her physician fill out a comprehensive, tablet-based questionnaire about her tick-borne illness symptoms, history, and treatment protocols.
collection
REVIEW
TRANSMISSION
analysis
How Clinical Data Gets Collected
questionnaire
collection
REVIEW
TRANSMISSION
analysis
Why Fund Tick Bytes?
What is tick bytes?
A shared clinical data repository that delivers faster, cheaper, and better data to researchers.
What problem will tick bytes solve?
why isn't that happening now?
— Low gov’t funding.
— Minuscule funding for tick-borne disease co-infections.
— The patient data pipeline is leaky.
— The cost/difficulty of collecting patient data.
Need more information?
Watch this video to learn how a large clinical data repository could significantly reduce the public health burden of tick-borne diseases.
In this video, Invisible International’s Education Co-Director, Christine Green, M.D, discusses the impact that a large clinical data repository could have on tick-borne disease research.
At Invisible International, we believe that there are effective treatments out there for Lyme patients. The problem is that these treatments could be underutilized as they haven’t been validated by the medical and scientific community. It’s difficult to understand what treatments are/aren’t working for patients without a reliable pool of data to make conclusions from thorough analysis. We hope to fix this by the evidence gathered through the Tick Bytes Clinical Data Repository.
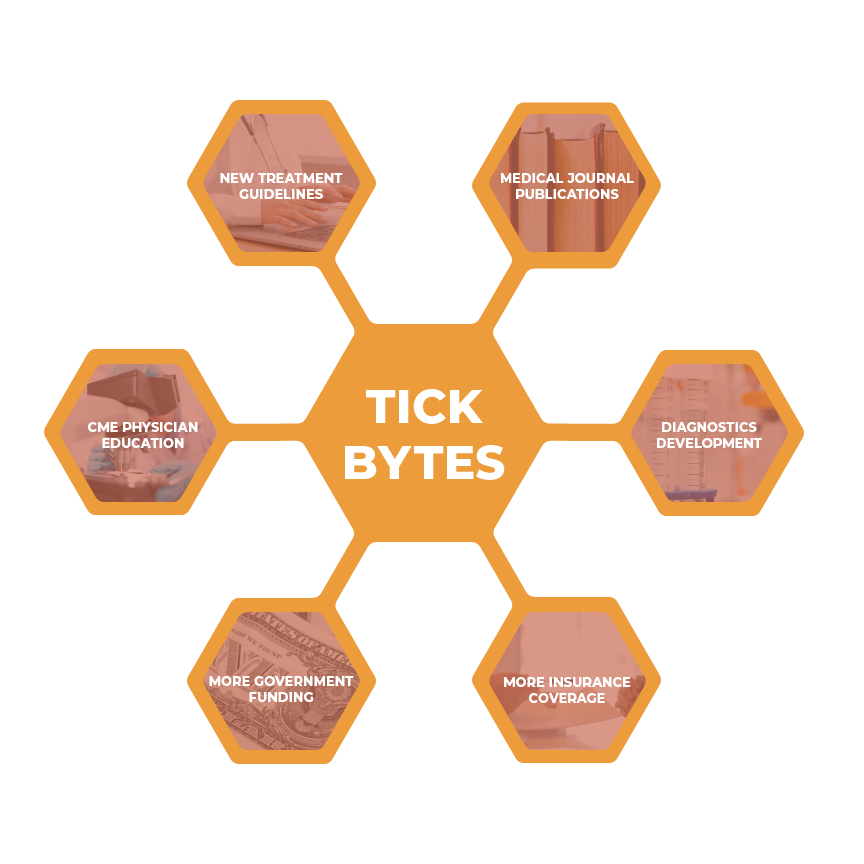
As Tick Bytes grows, professionals will be able to take our data and use it effectively to create the outcomes shown below.
questionnaire
Budget
We need $500,000 to get the first clinical site up and running. Here is a breakdown of how donations will be spent.
Study Site Grants - 47%
Direct funding for site-specific clinical data collection and investigator oversight
scientific team - 38%
Research leadership, outcomes research experts, biostatisticians, and data scientists
Research Operations - 8%
Data platform access and operations, site and grant management
Technology & Tools - 4%
Hardware, data storage, IRB fees
Advocacy & Communications - 3%
Conferences, meetings, outreach and advocacy efforts
No Data Found

