Announcing Invisible’s Clinical Guide for Lyme Neuroborreliosis
From the Desk of Dr. Nev Zubcevik
A letter to our supporters from Dr. Nev Zubcevik, Chief Medical Officer, on a new clinical tool that will help doctors better understand & care for patients with neurological Lyme disease.
Dear community members and supporters,
As a physical medicine and rehabilitation physician, my primary focus is on identifying the root cause of my patients’ illnesses. Only by addressing the underlying cause can we effectively rehabilitate our patient’s injuries. Throughout my years of practice, I have witnessed the devastating impact of untreated or under-treated Lyme disease infection on patients’ nervous systems. This destructive effect severely impairs their cognitive abilities, physical functioning, and overall quality of life. Our team at Invisible International has developed a clinical guide to assist clinicians in the recognition of neurological Lyme disease symptoms and subsequent diagnostic, testing, and treatment strategies to help diagnose and treat patients faster. We are grateful to donors like you who help fuel our work to pave the way for making sure every physician is a Lyme+ knowledgeable physician. To partner with us in developing and disseminating our education to physicians, please consider a tax-deductible donation today.
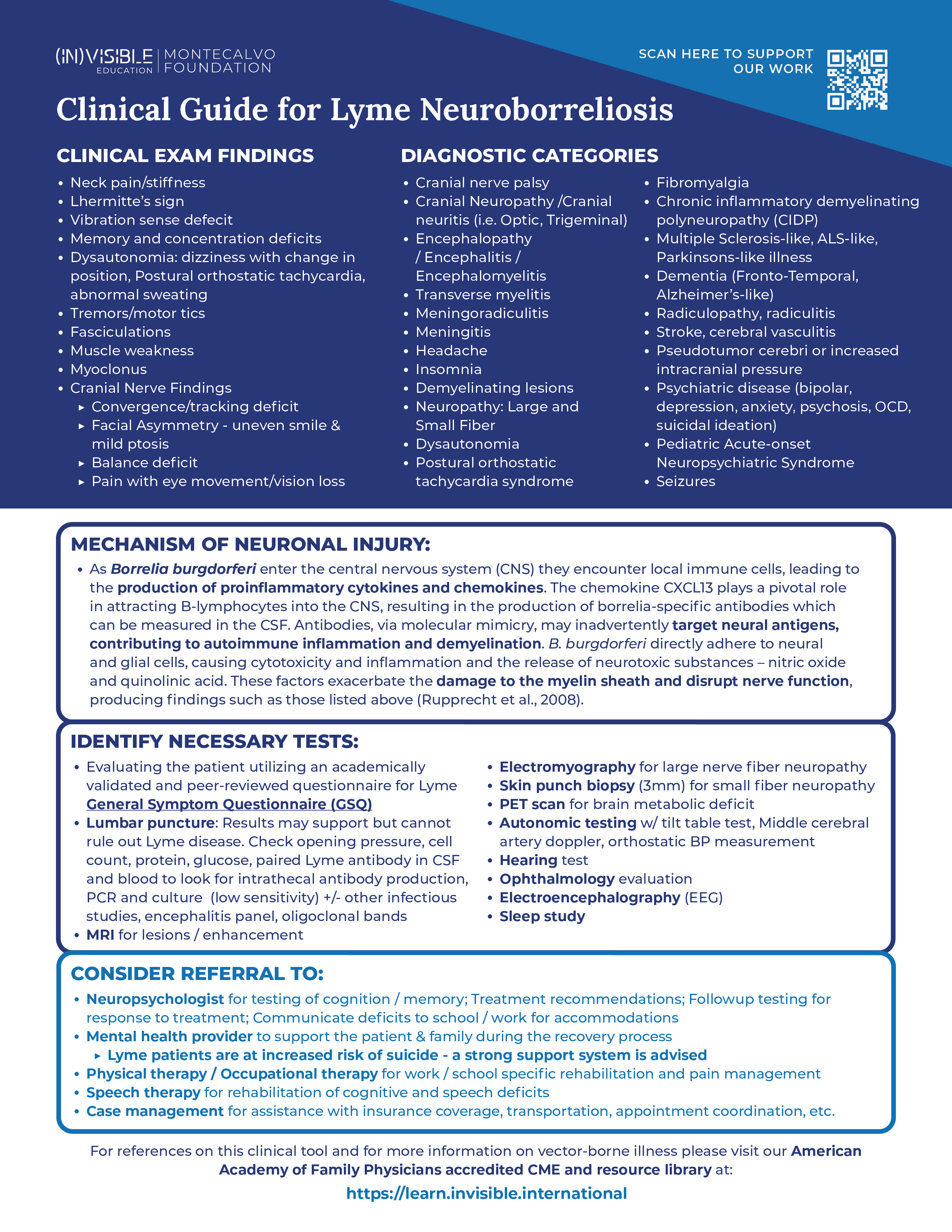
Lyme patients are at an increased risk of suicide
My deepest concern as a physician is that Lyme patients are extremely vulnerable as a population. Research has shown that Lyme patients face a heightened risk of suicide, primarily because their neurological injury remains largely invisible, causing immense suffering (Fallon et al., 2021). Understanding the clear mechanism of injury caused by the Lyme bacterium is crucial in explaining this invisible damage. By raising awareness among physicians and healthcare professionals about this mechanism, we can approach these patients with a clearer path to diagnosis and treatment.
Our study shows damage to the nervous system
In 2019, our team at Harvard conducted research and published the study “Association of Small Fiber Neuropathy and Post Treatment Lyme Disease Syndrome,” where we investigated the potential link between small fiber neuropathy (SFN) and post-treatment Lyme disease syndrome (PTLDS). Our findings provided both a biomarker of injury and a testing protocol that other physicians can use to objectify their patients’ neurological injury caused by Lyme disease.
In the study, we explored ten participants with a history of PTLDS, and through skin biopsies, we discovered evidence of SFN in all cases. Specifically, nine participants displayed sensory SFN with abnormal epidermal nerve fiber density, and seven individuals exhibited severe SFN. We observed autonomic dysfunction in all PTLDS participants. Additionally, our study revealed reduced cerebral blood flow in all PTLDS patients, suggesting cerebral hypoperfusion.
Our findings suggest that SFN and related dysautonomia may serve as objective markers for PTLDS. The assessment of small fiber density and autonomic dysfunction using skin biopsies and reflex testing could be valuable in therapeutic trials and offer physicians a clearer understanding of PTLDS and its associated symptoms, including cognitive impairment and brain fog.
The mechanisms like direct cytotoxicity by the spirochete, neurotoxic mediators during host-pathogen interactions, and triggered autoimmune reactions are likely to be involved in the pathogenesis of this neuronal injury.
The mechanism of neuronal injury in Lyme is clear
The article “The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation” by Rupprecht et al. (2008) is a crucial source of information that sheds light on the intricate mechanism of neuronal injury in Lyme disease. Lyme neuroborreliosis, caused by the spirochete Borrelia burgdorferi, can lead to neurological manifestations, including painful meningoradiculitis and cranial or peripheral neuritis. Understanding the pathogenesis of this condition is essential for effective management and treatment.
The infection process begins with the spirochetes entering the tick’s salivary glands during feeding and subsequently invading the host’s skin, leading to a local infection called erythema migrans. During the second stage of Lyme disease, the spirochetes can spread to various organs, including the central nervous system (CNS), resulting in neurological complications.
The spirochetes employ various strategies to evade the host’s immune system. They downregulate immunogenic surface proteins, such as OspA and OspC, to minimize their recognition by immune cells. Additionally, they express complement-neutralizing proteins and induce anti-inflammatory cytokines to suppress the host’s immune response. These mechanisms enable the spirochetes to establish infection and persist in the host.
Once the spirochetes enter the CNS, they encounter local immune cells, leading to the production of proinflammatory cytokines and chemokines. The chemokine CXCL13 plays a pivotal role in attracting B-lymphocytes into the cerebrospinal fluid (CSF), resulting in the production of borrelia-specific antibodies. This immune response, however, can also contribute to the neuronal injury.
The neurological dysfunction observed in Lyme neuroborreliosis may result from multiple factors. The spirochetes can directly adhere to neural and glial cells, causing cytotoxicity and inflammation in the surrounding tissues. Furthermore, they induce the release of neurotoxic substances, such as nitric oxide and quinolonic acid, exacerbating the damage. Additionally, the immune response may lead to an autoimmune reaction, with antibodies targeting neural antigens due to molecular mimicry, further contributing to inflammation and demyelination.
The demyelination process is particularly significant as it can disrupt nerve function and result in various neurological symptoms. Damage to the myelin sheath, the protective covering of nerve fibers, can lead to muscle weakness, numbness, tingling, and coordination difficulties.
What we are doing and how you can help
The mechanisms discussed, including immune evasion, inflammation, and demyelination, contribute to the complex clinical picture of this condition. Understanding these processes is crucial for developing targeted therapies to mitigate nerve injury and promote recovery in patients with Lyme neuroborreliosis. We must do better to educate the medical system about this mechanism of injury. With this information, the stigma will disappear, and the patients will be listened to and treated properly. Insurance companies will follow this by covering treatments.
Education leads to meaningful and lasting change. And we are paving the way.
Just in the last 6 months, we have educated physicians via the Montecalvo Education Platform for Vector-Borne Illness to impact over 750,000 patient visits. Our virtual courses have been viewed over 14,000 times. This work is only possible with your support: we rely on gifts from donors like you to make sure no Lyme patient is left behind. Your donations help us expand programming, send our team to conferences, and help us develop educational guides. Please consider making your tax-deductible donation today.
From all of us here at Invisible,
With gratitude,
Nevena Zubcevik, DO
Chief Medical Officer
Invisible International
References:
- Fallon BA, Madsen T, Erlangsen A, Benros ME. Lyme Borreliosis and Associations With Mental Disorders and Suicidal Behavior: A Nationwide Danish Cohort Study. Am J Psychiatry. 2021 Oct 1;178(10):921-931. doi: 10.1176/appi.ajp.2021.20091347. Epub 2021 Jul 28. PMID: 34315282.
- Novak P, Felsenstein D, Mao C, Octavien NR, Zubcevik N. Association of small fiber neuropathy and post treatment Lyme disease syndrome. PLoS One. 2019 Feb 12;14(2):e0212222. doi: 10.1371/journal.pone.0212222. PMID: 30753241; PMCID: PMC6372188.
- Rupprecht TA, Koedel U, Fingerle V, Pfister HW. The Pathogenesis of Lyme Neuroborreliosis: From Infection to Inflammation. Mol Med. 2008 Nov-Dec;14(11-12):205-12. doi: 10.2119/2007-00091.Rupprecht. PMID: 18787810; PMCID: PMC2270991.


